GIBBS FREE ENERGY

Gibbs Free Energy
Definition:Gibbs free energy (G), named after the American physicist Josiah Willard Gibbs, is a thermodynamic potential that measures the maximum reversible work that can be done by a system at constant temperature (T) and pressure (P). It is particularly useful for understanding and predicting whether a chemical reaction will occur spontaneously under these conditions.
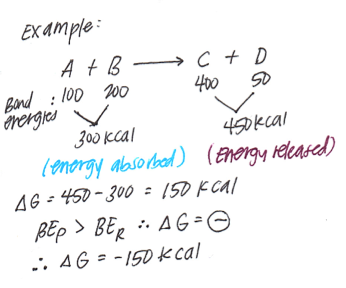
Mathematical Expression:The Gibbs free energy is mathematically defined as:
�=�−��G=H−TS
Where:
- G is the Gibbs free energy.
- H is the enthalpy of the system (the total heat content).
- T is the absolute temperature in Kelvin.
- S is the entropy of the system (a measure of disorder or randomness).
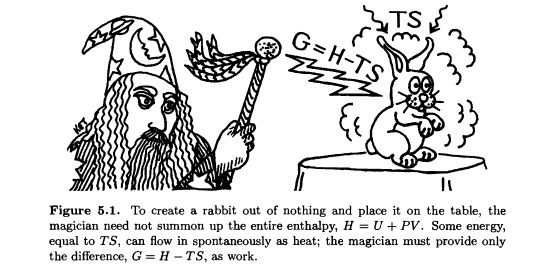
Explanation:
- Enthalpy (H): Enthalpy represents the total internal energy of a system, including both the internal energy (U) and the pressure-volume work (PV). It is a measure of the heat content of a system. When a chemical reaction occurs at constant pressure, the change in enthalpy (ΔH) corresponds to the heat gained or lost by the system.
- Entropy (S): Entropy is a measure of the degree of disorder or randomness in a system. It quantifies the number of possible microscopic arrangements of particles in a system. In thermodynamics, it is often associated with the concept of the dispersal of energy or the tendency of systems to move toward a state of higher entropy.
- Temperature (T): Temperature is an absolute measure of thermal energy. In the context of Gibbs free energy, it should be expressed in Kelvin.
Significance of Gibbs Free Energy:
The key concept related to Gibbs free energy is spontaneity. A chemical process or reaction will be spontaneous if the change in Gibbs free energy (ΔG) is negative:
Δ�<0ΔG<0
In this case:
- If ΔG is significantly negative, the reaction is spontaneous and exergonic, meaning it releases energy. The system tends to move towards a more stable state.
- If ΔG is significantly positive, the reaction is non-spontaneous and endergonic, meaning it absorbs energy. The system does not tend to occur naturally in this direction.
- If ΔG is close to zero, the system is at equilibrium, and there is no net change in the system.

In summary, Gibbs free energy is a crucial thermodynamic parameter that helps us understand the spontaneity and direction of chemical processes under constant temperature and pressure conditions. It tells us whether a reaction will proceed naturally or if it requires external energy input to occur.
THINGS YOU MAY NOT KNOW: GIBBS FREE ENERGY FORMULAS AND HOW A LAYMAN CAN USE THIS IN DAILY LIFE.
THINGS YOU MAY WANT TO SAVE: DONT CLUTTER YOUR MIND WITH THESE COMPLCATED MATH FORMUlAS ALLOW AI BLOCKCHAIN TO TAKE OVER.
ZENTRAVELER SAYS: My math teacher Snuffy Clark failed to teach our class the concept of GIBBS FREE ENERGY No wonder I flunked college math?
From here to Infinity is a relatively short ride! The next leg takes eons and eons as you fly through the Barycentric Dynamical Time Zone! …and on and on and on. Follow the Zentraveler Newsletter often for Travel, Health and Zen-like stories and such. Where else can you get a THREE IN ONE NEWSLETTER FOR THE PRICE OF FREE.

ZENTRAVELER IS A PERSONAL NEWSLETTER, DESIGNED TO GIVE TRAVEL, HEALTH, WRITING AND HUMOR INCLUDING HELPFUL HINTS WITH A ZEN LIKE QUALITY.
PLEASE CHECKOUT MY NEW VIDEO PODCASTS AT ZENTRAVELER ON YOUTUBE...THANKS
